Drive innovation, advance healthcare
GDPR compliance is crucial for Life Sciences organizations, but the process can be complex. Simplify and automate your GDPR compliance efforts, and focus on what really matters: driving innovation and advancing healthcare.



















GDPR in Life Sciences
In the life sciences sector, GDPR compliance is crucial due to the nature of handling sensitive health data in activities such as clinical trials and research. Researchers and companies in life sciences must prioritize GDPR adherence, ensuring robust documentation and implementation of GDPR principles throughout the organization. This includes considerations for data minimization, integrity, confidentiality, and addressing various data subject rights.
Privacy challenges in Life Sciences
Data processing
Personal and special category data is being collected and processed
Data transfers
Personal data might be subject to an international transfer
Data subject rights
Provide the data subjects with accurate information on their rights
Data breaches risk
In need of high security measures to prevent and detect a breach
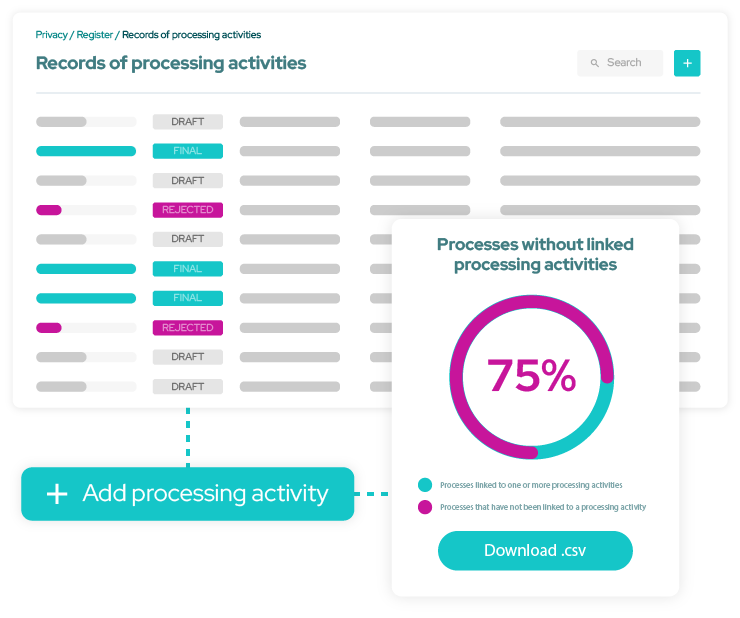
Simplify privacy management
Ensure GDPR compliance
Automate your Records of Processing Activities and respond to data subject requests in a timely fashion.
Evaluate the impact of your activities
Assess and mitigate privacy risks in data processing activities, and comply with regulations to protect individuals’ privacy with Data Protection Impact Assessments (DPIAs).
Mitigate security risks
Build an effective security strategy, keep a clear overview and manage all the appropriate security controls, execute Vendor Assessments and keep track of stakeholder contracts.
Demonstrate accountability
Have the correct information readily available in one single platform in case of an audit.

eBook
DPO's insider guide to clinical trials
Our ebook is a practical guide, crafted to assist you in navigating the captivating yet intricate world of privacy, with a specific focus on clinical trials.
What's more
In the dynamic landscape of data protection and privacy, RESPONSUM emerges as a robust solution designed to revolutionize how organizations navigate and manage their privacy responsibilities. Our privacy management software seamlessly integrates a range of powerful features, ensuring your compliance journey is not only efficient but also effective.
Automation
Work more efficiently with automated tasks instead of focusing on repetitive administrative work.
Linked items
Don’t miss any important information. With linked modules you have all the information you need at your disposal.
Accountability & availability
No more spreadsheets. Have the correct information readily available in one single platform in case of an audit.
Simplification
Translate complex Privacy legislation requirements into easily understandable and applicable language through our guided workflows.
Education
Raise and maintain your organization’s awareness to the highest level through simulations and online trainings.
Usability
Get the all-in-one user-friendly tool, that guides the user throughout each process.
Discover the easy route to GDPR compliance
Fill out the form and find out how RESPONSUM can help you achieve compliance. We’ll get back to you within a couple of days!
* RESPONSUM is committed to protecting and respecting your privacy. We will only use your personal information to administer your account and to provide the products and services you requested from us. From time to time, we would like to contact you about our products and services, as well as other content that may be of interest to you. If you consent to us contacting you for this purpose, please tick above to confirm we may contact you. You can unsubscribe from these communications at any time. For more information on how to unsubscribe, our privacy practices, and how we are committed to protecting and respecting your privacy, please review our Privacy Policy (www.responsum.eu/privacy-statement/). By clicking submit above, you consent to allow RESPONSUM to store and process the personal information submitted above to provide you the requested communication.


